
So, you are interested in buying or using the KN95. But there is so much (mostly negative news) out there about it. It is confusing. It is scary. Is it safe?
My team and I continue to get emails, calls, and texts about the KN95 and the goal of this post is to address three key questions or issues that come up.
Is the KN95 banned? Is it counterfeit? Have the FDA or Health Canada banned all KN95s?
So here is what happened. When the COVID-19 pandemic began, there was a huge shortage of masks, specifically the N95. As such, governments and health authorities like the FDA or Health Canada lifted certain requirements to allow for respirators and face masks to be distributed quickly, such as the KN95.
The KN95 is a Chinese standard mask that has similar properties to the N95, in terms of blocking bacteria and particulate (fancy word for little things like dust) by at least 95%.
When it was clear governments needed supply and were accepting the importation of the KN95, suppliers started importing this particular mask by the tens of millions. Everyone under the sun, including yours truly, were sourcing any and all suppliers in China.
So fast forward a few weeks into April and all these KN95s start arriving. Everyone is happy at first because they got the “N95.” What many glanced over was that these were the KN95. That means it is close to the N95 because it also blocks out 95% of the bacteria/particulate (hence the 95 in the name), but it does not look like the N95 that people have come to know, most notably by brands like 3M.
Once enough people start looking at these KN95s, they start to question. Amongst the concerns, many of these KN95s from hundreds of different manufacturers were sent to 3rd party testers in North America.
Funny thing happens. Many of them failed!! How can that be?! These are supposed to be comparable to the N95. Governments bought millions on these and spent billions!
I It turns out many of these masks that claimed to block 95% of the bacteria or particulate did not meet that standard. The Governments of Canada and the USA realize they just blindly bought tens of millions of masks and were embarrassed.
To save face and more importantly to protect people, these governments put together a list of manufacturers that they have tested that DO NOT meet the standards set by the N95 or the KN95.
While some are in fact counterfeit because they are pretending to be a brand when they are not, the other ones that do not meet the 95% threshold are not in fact banned. They are just not allowed to be sold to the medical community (we will get to that later) because they are under the 95% filtration requirement.
It is only specific manufacturers that are considered counterfeit or banned. It is not all KN95 brands.
So as long as the KN95 brand and the particular mask meet or exceed the standards via proven 3rd party data, it is allowed to be sold to both medical and non-medical groups. A manufacturer DOES NOT need to be on the approved list – these lists are guidance for those that have been tested. However, DATA always rule: so long as the data for the KN95 is valid, it is allowed to be imported and sold to both the medical and non-medical community., even if a manufacturer is not on an approved list by the FDA, CDC or Health Canada
Isn’t the KN95 supposed to be a Medical Product? How come all the packaging says Non-Medical.
The KN95 is in a fact both a medical and non-medical product, just like the N95 but it is a more complicated explanation.
Some backstory. By its nature, the N95 is not typically used in a medical setting. Ask many doctors or dentists and they may not have worn a N95, KN95 or similar product until COVID. Some may have worn one during the last pandemic, SARS, but it is not normally a product used in a medical setting.
In ‘normal times,’ The N95 is a mostly an industrial-use mask. As such, instead of the FDA or Health Canada, which are health-related arms that certify the N95, this particular mask does not need to be certified by these bodies to be imported and distributed. But there is a catch: it can’t be sold or marketed as a medical device.
Due to the pandemic, even though the N95 and related masks like the KN95 are meant for non-medical use, they have been temporarily allowed for sale and use within a medical setting because of their ability to block 95% of bacteria (known as BFE) and particulate (PFE),
The N95 and he KN95 actually have medical versions too, but they are not currently available due to the shortage of masks; and as such both the N95 and KN95 non-medical masks have been approved for use in a medical setting so long as certain standards are met, mainly the 95% particular filtration efficiency (PFE).
But here is what happens.
Some of the N95 and KN95 that were imported from China (and other countries) did not meet the basic criteria like blocking 95% of the PFE. Because of this, the KN95 starts to get a bad name and the Chinese Government acts fast.
In the middle of April, the Chinese Government clamps down hard on all mask exports out of China. Rules start changing by the hour on exporters. So many ‘fly-by-nights’ were shipping KN95 and other protective equipment that the Chinese Government wanted to put in procedures ASAP that would help ensure that quality product was being made and exported out of the country.
The Chinese Government made immediate changes, like mandating that only those companies that have an approved medical license by the National Medical Products Administration (NMPA) could export medical products. Because the original use of the KN95 or N95 was non-medical, any non-medical products like these masks, whether made by a medical factory or not, MUST state non-medical on the packaging; even if the end use was medical or sold to medical facilities.
These changes sent manufacturers, suppliers, and customs officers into a tailspin of repackaging and paperwork to comply with the immediate changes in China. Shipments were delayed and planes left empty. Some of these changes included removing any FDA or CE stamps on products – again, all of this was done by the Chinese Government to help ensure that the products being shipped around the world met (at minimum) the N95 or the KN95 standard, which is actually known as GB2626-2006.
Since the N95 and KN95 are in fact non-medical products, everyone wanted to make sure it was clearly stated – sort of a “use at your own risk”, while also saying “we believe this is a viable option given the current situation”. So that is why the product states Non-Medical.
Oliberté factory partners meet these standards and if you are unsure whether the KN95 complies, you should ask the place where you purchased them to provide the lab reports to verity this. Anyone importing these should have access to that. Some, like Oliberté, even do their own testing as an additional verification. A word of caution: make sure the results you receive are clear (nothing blurred out) and are within a reasonable time frame (90 days) because each batch of material can be different and you do not want to use data from a year ago.
The Standard: N95 and KN95
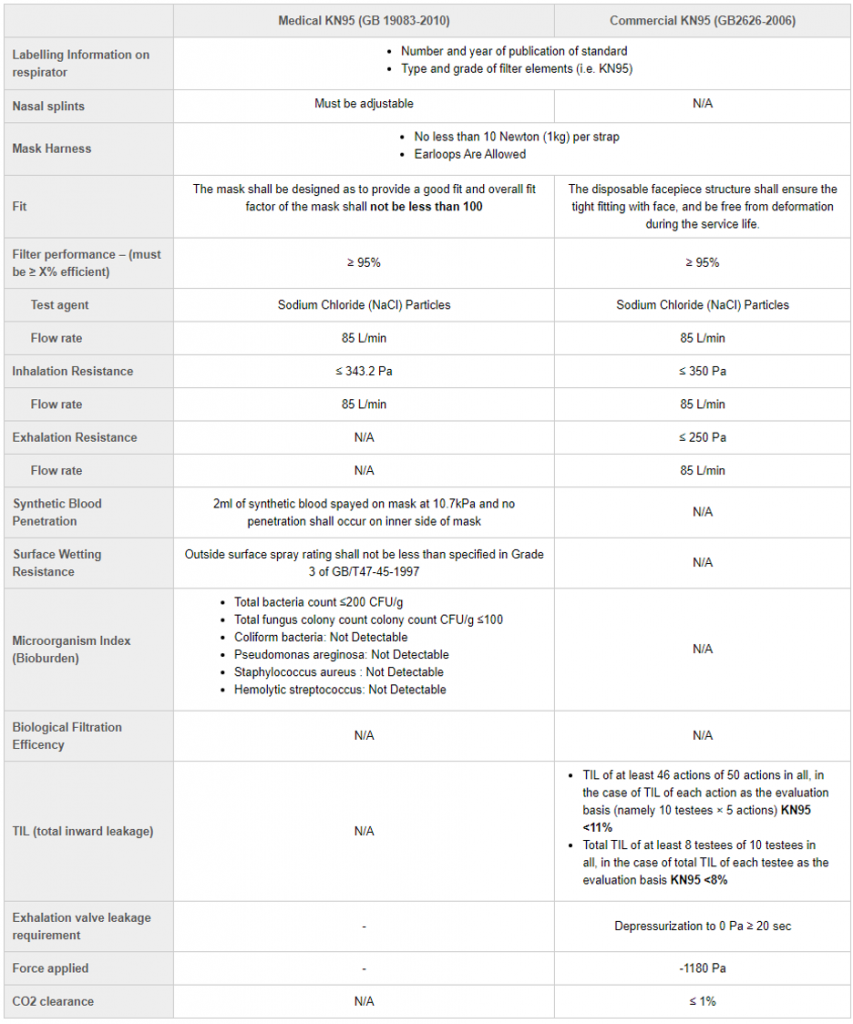
Is the KN95 mask faulty? My fit test person said these masks are not compliant. NIOSH said it is not approved too.
This is something we hear especially from those in the medical or dental field. Many people in these professions are regularly exposed to little things called aerosols that need to be blocked. Certain professions need what is called ‘a perfect seal.’, which means nothing can get in through the top or bottom of the mask and potentially harm you, even if your face moves.
Here is where we need to talk about the design of the KN95 compared to the N95, specifically a NIOSH approved N95. FYI – NIOSH stands for National Institute of Occupation Safety and Health.
To be certified as a NIOSH approved masks, amongst many standards like the 95% filtration of PFE and BFE, it MUST pass a fit test. NIOSH only approves N95 masks that have head bands around the back of your head as a strap construction.
The KN95 as a result will NEVER be NIOSH approved as it is an ear-loop construction. An ear-loop construction means that the straps go around the ears (like a standard disposable face mask). As such, it will rarely pass a fit test. As the mouth, jaw or nose move around, it will create gaps between the mask and your face.
This does not mean these masks are faulty. The KN95 was never meant to pass a fit test. It is called a KN95 because it meets all the key standards that are required related to material and filtration of the N95. The fit test is a requirement by NIOSH, which is not medical body. However, NIOSH is an important partner with the FDA during this pandemic, and as a result used by Health Canada to ensure key standards are met.
While the KN95 will likely not pass a standard fit test because of the ear loop construction, that is not the only reason. The KN95 one-size fits all and does not come in multiple sizes like some N95 masks. Again, that is part of the reason it is not likely to pass a fit test. No two faces are the same. It does not mean it’s faulty. The KN95 is just a different design. Saying that, there are some tricks that you can do to help ensure a better fit with the KN95 and many medical and dental groups that we work with have had success with these strategies. Some include tying knots in the ear loops to tighten them. One of the more successful ones we have seen is using an ear extension that allows for the masks to be pulled tighter. There are different names for these like mask extenders or ear extenders and we will be offering them soon. While this still will not guarantee a 100% fit for everyone, it can help.
Fitted or not, it is important to reiterate that these KN95 masks, while not intended for medical use, can be used in a medical setting if they meet the correct standards. Given the circumstances of COVID-19 where supply of masks and, in particular the N95, is extremely limited, the KN95 is a viable option when understood correctly.
There are cases where 100% fit is required, but in certain cases where fit is a guideline, or an ideal, using the KN95 in combination with other PPE like 3-ply face masks and a face shield, is something we are seeing in practice medically and non-medically. Why? The ability to use the KN95 is the ability to work again and earn income. The KN95 allows us to protect others while protecting ourselves.
Starting July 1st, 2020 the updated standard for the KN95 is GB2626-2019, what does that mean for my mask that says GB2626-2006?
In December of 2019, the Standardization Administration of China Standard updated the standards for the KN95. This standard for the KN95 is called GB2626-2019 and was to go alongside the GB2626-2006. The launch date for this is July 1st, 2020.
In this upgrade there are some additional standards for the KN95 to meet, but the GB2626-2006 is still a valid KN95 standard and can be used safely.
The GB2626-2006 is a an approved standard that is recognized by Health Canada, the FDA and CDC.






